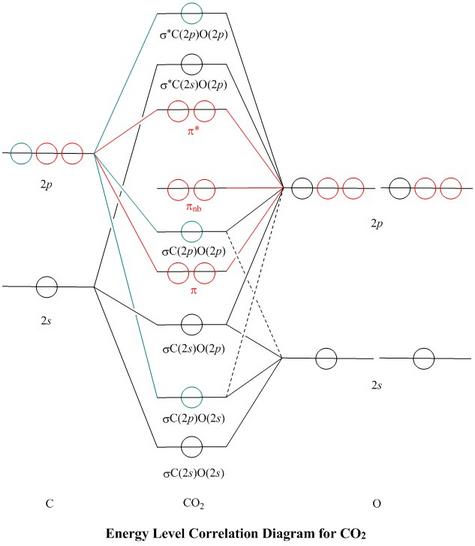
how to draw molecular orbital diagram for co2
If non-linear y axes of outer atoms point to central atom3. Based on the rules of the Lewis Structure all 16 electrons are filled upon bond formation but the nonbonding orbitals remain vacant as in the case of CO2.

Molecular Orbital Diagram Of Polyatomic Co2 Molecules Chemical Bonding Molecular Structures Youtube
Consider the h 2 molecule for example.
. Note that the n 1 level only has s orbitals the n 2 level only has s and p orbitals and the n 3 level only has s p and d orbitals. The molecular orbitals are no longer symmetrical and the energies of the bonding molecular orbitals are. The applications of the MO theory extend beyond the limitations of the Valence Shell Electron Pair Repulsion VSEPR model and the Valence Bond theory.
Molecular orbital diagram as a non-bonding molecular orbital. Home Structure and Bonding Atomic Orbitals Molecular orbitals in Carbon Monoxide CONTROLS Click on the CO molecular orbitals in the energy level diagram to. These are UVVisible Infra-red IR and Nuclear Magnetic Resonance NMR spectroscopies.
Find the characters of the reducible representationfor the combination of. Answer 1 of 3. See Resources for a diagram showing the filling order.
Introduction to Molecular Spectroscopy. The first major step is understanding. How to draw molecular orbital diagram for heteronuclear molecules.
Next well see that symmetry will help us treat larger. Molecular Orbital MO Diagram In a Molecular Orbital Diagram the 2s orbital of oxygen is nonbonding because of the high energy difference between carbon and oxygen atoms. Oct 26 2016 3 min read.
The course introduces the three key spectroscopic methods used by chemists and biochemists to analyse the molecular and electronic structure of atoms and molecules. Thus we take 10 atomic orbitals and generate 10 molecular orbitals in accordance with the conservation of orbitals. Draw the orbital diagram for ion Co 2.
It is a linear molecule. Molecular Orbital Theory Walsh diagram The Walsh diagram shows what happens to the molecular orbitals for a set of molecules which are related in structure. Molecular Orbitals for Larger Molecules 1.
It is a linear molecule. Sp Hybrid Orbitals in BeH2 1. High-spin octahedral d7 has LFSE o.
Can be accommodated in the metal d orbitals. Draw the orbital diagram for the ion Co2. Assign x y z coordinates z axis is principal axis.
Atomic orbitalsAOs linearly combine with each other to form equal number of molecular orbitals MOs. Draw the orbital diagram for the ion Co2. A good way to visualize the orbitals and the filling of orbitals is to draw energy level diagrams such as figure20 through figure23.
Summary MO Theory LCAO-MO Theory is a simple method for predicting the approximate electronic structure of molecules. As sp orbitals are hybridized to form the bonds CO2 has an sp hybridization. D0 ions d7 ions Fe1 Ru1 Co2.
The Aufbau principle tells you that the lowest-energy orbitals fill first but the specific order isnt sequential in a way thats easy to memorize. Determine point group of molecule if linear use D2h and C2v instead of Dh or Cv 2. The content is presented using short focussed and.
A molecular orbital diagram or MO diagram is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals LCAO molecular orbital method in. Watch the video solution for the question. The Lewis structure shows that the beryllium in BeH 2 makes 2 bonds and has no lone pairs.
So you must always be flipping it back and forth 4 the number of nodes in your molecular orbitals must always begin at 0. Based on the amount of orbital overlap the relative changes in energy differ going from the atomic orbital to the molecular. Half of the molecular orbitals MOs having energy lower than the atomic orbitals are called.
The molecular Geometry of any compound is based on the arrangement of atoms electron pairs and bonds. Use the buttons at the top of the tool to add orbitals in order of increasing energy starting at the bottom with theOct 05 Also Cobalt orbital diagram example problem. In this case the difference is the H-X-H bond angle which decreases from 180 o to 90 o Molecular Orbital Theory Walsh diagram Water 1045 X H H H O H.
The lewis structure shows that the beryllium in beh 2 makes 2. There are a total of 6 electrons to add to the molecular orbital diagram 3 from boron and 1 from each hydrogen atom. The molecular orbital MO theory is a powerful and extensive approach which describes electrons as delocalized moieties over adjacent atoms.
How to draw molecular orbital diagram for CO2 By signing up youll get thousands of step-by-step solutions to your homework questions. Use the buttons at the top of the tool to add orbitals in order of increasing energy starting at the bottom with the. You have now 2 electrons left.
Atomic orbitals must have the proper symmetry and energy to interact and form molecular orbitals. At the next level of detail I would point to the spectroscopic data which has been around for over 100 years as strikingly direct evidence for molecular orbitals. Molecular orbital MO theory explains the construction of molecular orbital diagram on the basis of following main points.
Now mo diagrams are only simple for elements of the second row of the periodic table celi through cene. How to Build Molecular Orbitals. Here in CO2 both Oxygen atoms form sigma bonds with the central carbon atom and complete their octet.
Drawing molecular orbital diagrams is one of the trickier concepts in chemistry. Photoelectron spectroscopy provides useful information on the energies of atomic orbitals. The angular overlap diagrams for the molecular orbitals with high d orbital.
The atomic orbitals of oxygen are uniformly lower in energy than the corresponding atomic orbitals of element C because of the increased stability of the electrons in oxygen. Molecular Orbital Diagrams simplified. Watch the video solution for the question.
So your first molecular orbital should have 0 nodes and then increase with increase by one with each increasing energy level so the more energy levels you have you would just increase the number of nodes by one each. Draw the orbital diagram for ion Co 2.
What Is The Molecular Orbital Energy Diagram Of Co Quora

Using The Molecular Orbital Model Write E Clutch Prep

Molecular Orbitals For Carbon Monoxide
Mo Diagrams For Linear Triatomic Molecules Chemistry Libretexts
Answer In Inorganic Chemistry For Lontum Rodrique 112252

Draw The Mo Diagram For Co2 2 And Identif Clutch Prep

Molecular Orbital Diagram Of Polyatomic Co2 Molecules Chemical Bonding Molecular Structures Youtube

Co Mo Diagram Chemistry Lessons Chemistry Classroom Teaching Chemistry

Co2 Lewis Structure Hybridization Molecular Geometry And Mo Diagram Techiescientist

Mo Diagram Of Co2 Preparation Of Gate Csir Net Uset Set Exam Youtube

Figure S6 Molecular Orbital Mo Diagram For The Valence Mos Of Ibr Download Scientific Diagram

Draw The Orbital Diagram For Carbon In Co 2 Showing How Many Carbon Atom Electrons Are In Each Orbital Study Com
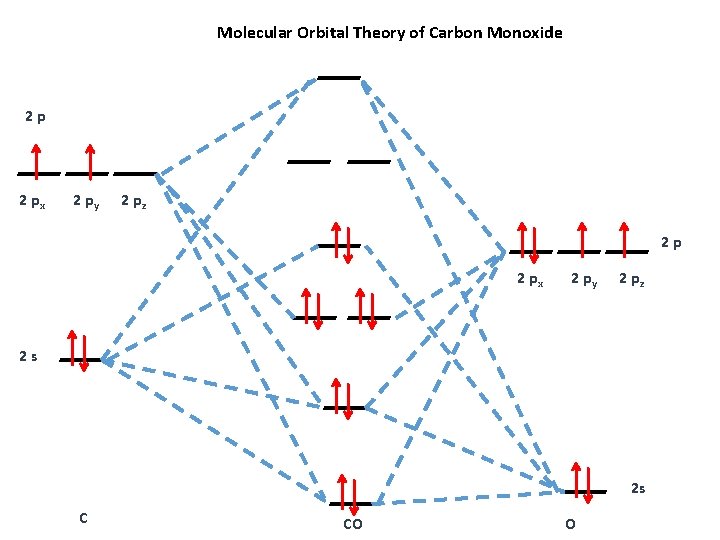
Molecular Orbital Theory Or When Electrons Dont Like

8 Drawing Molecular Orbital Diagrams Flux Science
Draw The Diagrams For No 2 No 2 And No 2 The Homo Of No 2 Shows It Is Somewhat Anti Bonding Would You Expect The Nonbonding Electron Pairs On Nitrogen Or Oxygen To Be More Reactive

Molecular Orbital Diagrams In Latex Tex Latex Stack Exchange